Safe use of medical equipment
المقالات >
Safe use of medical equipment
م.مطلق العنزي
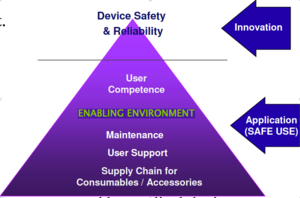
Overview and recommendation
A medical device can range from a simple wooden tongue depressor or stethoscope to the most sophisticated implants or medical imaging devices. In general, a medical device is an instrument, apparatus, or machine used to prevent, diagnose or treat disease. It also serves to detect, measure, restore or modify the structure or function of the body for a given health purpose.
one of the important components of patient safety is medical device safety. By increasing medical device safety, patient safety increases, need to ensure and promote quality, safety, and effectiveness of medical devices, and to minimize the risks associated with the usage, transportation, and storage of medical devices at healthcare facilities, by defining the medical equipment hazard and the role of each persona dealing with medical equipment, and we need to be aware of the best practicing and standers to manage the medical equipment.
Medical Equipment Hazard
Medical equipment can present a range of hazards to the patient, the user, or to service personnel.
Many such hazards are common to many or all types of medical electrical equipment, whilst others are peculiar to particular categories of equipment.
The root causes for injuries Mechanical Hazards involving medical equipment include Human Error, Faulty Equipment Design & Poor Maintenance.
Common Hazards of medical equipment :
- Mechanical Hazards
- Risk of Fire or Explosion
- Absence of Function
- Excessive or Insufficient Output
- Infection
- Misuse and human error
- Risk of Exposure to Spurious Electric Currents
- Radiation hazard
Safe use of medical equipment
Optimum safety and performance require cooperation among all involved in the life span of a medical device the MOH, the manufacturer ,SFDA, the importer/vendor, the user, and the public , each has a specific role to play in this risk management.

Responsibilities and Roles
Healthcare providers shall:
- Ensure that all relevant medical devices are approved by SFDA.
- Ensure that all clinical studies related to medical devices that
- are carried out in the healthcare facilities have been registered
- with the SFDA and obtain “No Objection Letter”.
- Deal only with medical devices distributors licensed by SFDA.
- Report to Biomedical Department in your facility for any adverse event caused by medical devices.
- Ensure that all necessary information and documentation accompanying a medical device, including instructions for use, have been provided.
- Ensure to be removes the recalled medical device from use or implements the recommended action for any affected device/product mentioned in the Recalls.
- Ensure the users of the medical devices are properly trained based on the manufacturer requirements before operating and maintaining the medical device.
- Ensure that the medical device is used according to the intend of use.
- Not re-use any medical device designed for single use only.
- When transferring a device to be used in another location or department, a transfer form shall be filled The form shall contain at least the device description and asset number, present location, new location, date of transfer, must be sent to the BME department to update the device’s record in order to keep track of the device for the purpose of the device’s maintenance, utilization, and future needs assessments.
- Ensure all quality check and validation of medical equipment Done as recommendations.
BME department shall:
- Ensure availability of test equipment, and ensure they are calibrated as per manufacturer requirements by an authorized lab.
- Ensure availability of maintenance management system and inventory management system to collect, store, organize, analyze, and report medical device data for healthcare provider. The maintenance management system shall include record for each device.
- Source appropriate and genuine spare parts for maintenance based on manufacturer service manuals and technical bulletins.
- Provide mechanisms to avoid failures or breakdowns of the device during patient treatment, diagnosis or therapy.
- Be proactive in the identification of possible points of failure of device related services and develop contingency plans before the catastrophic event or possible incident.
- Plan, with the assistance of the device supplier, for appropriate inventories of spare parts to minimize downtime.
BME/BMT shall:
- Assure that the status of the device is matching the manufacturer specifications and comply with SFDA regulations.
- Take into account the manufacturer instructions for CM and PPM.
- Ensure and maintain the accuracy of the maintenance management system and inventory information.
- Ensure that all relevant medical devices are approved by SFDA.
- Ensure that medical devices distributers dealing with are licensed by SFDA.
- Ensure the availability of manuals (user, operation, instructions for use , service, spare part list, lists of tool and test equipment required, circuit diagram, planned preventive maintenance manual and checklist as per manufacturer’s requirements).
- Review of published hazard and recalls data to identify any ongoing technology issues
- Apply the evaluation criteria for all suppliers of medical devices
- Ensure the availability of the decontamination, cleaning, disinfection and sterilization procedures, ensuring the healthcare provider is able to reprocess in line with the manufacturer’s instructions
- Ensure the availability of disposal instructions
- Only trained personnel shall perform the PPM.
- After performing PPM, a device’s performance and function tests shall be carried out , In all cases, this shall include a safety test and safety inspection as part of the PPM.
Medical devices have the potential to improve the quality and safety of healthcare. However, their introduction and use also carry risks for the safety of patients and staff ,Studies and all recommendations also show the importance of improving the safety and safe use of medical devices in various settings , we can maintain the high level of safe use by following all the recommendation and international standers and be sure all end user get the proper training and also the biomedical staff monitoring the medical equipment performance to be sure the safe outcome form the medical technology.
There are different organizations which play a major role in maintaining medical devices safety. These organizations set policies and standers to ensure safe use of medical technologies. One of leading organizations is ECRI (Emergency Care Research Institute). ECRI is a non-profit organization that test medical devices for safety. In addition, ECRI releases ECRI recall list that has all information of risky medical equipment and the necessary precautions to take to eliminate the risk. Another important organization is SFDA (Saudi Food and Drug Authority). SFDA regulate, oversee, and control medical devices, as well as set mandatory standard specifications for medical devices. Moreover, the SFDA spread consumers awareness on all matters related to medical devices and all other products and supplies.
